Clinical Programs
CLINICAL PROGRAMS
Equilibra Bioscience obtained exclusive technology licenses of anti-APC mAb from Oklahoma Medical Research Foundation (OMRF).
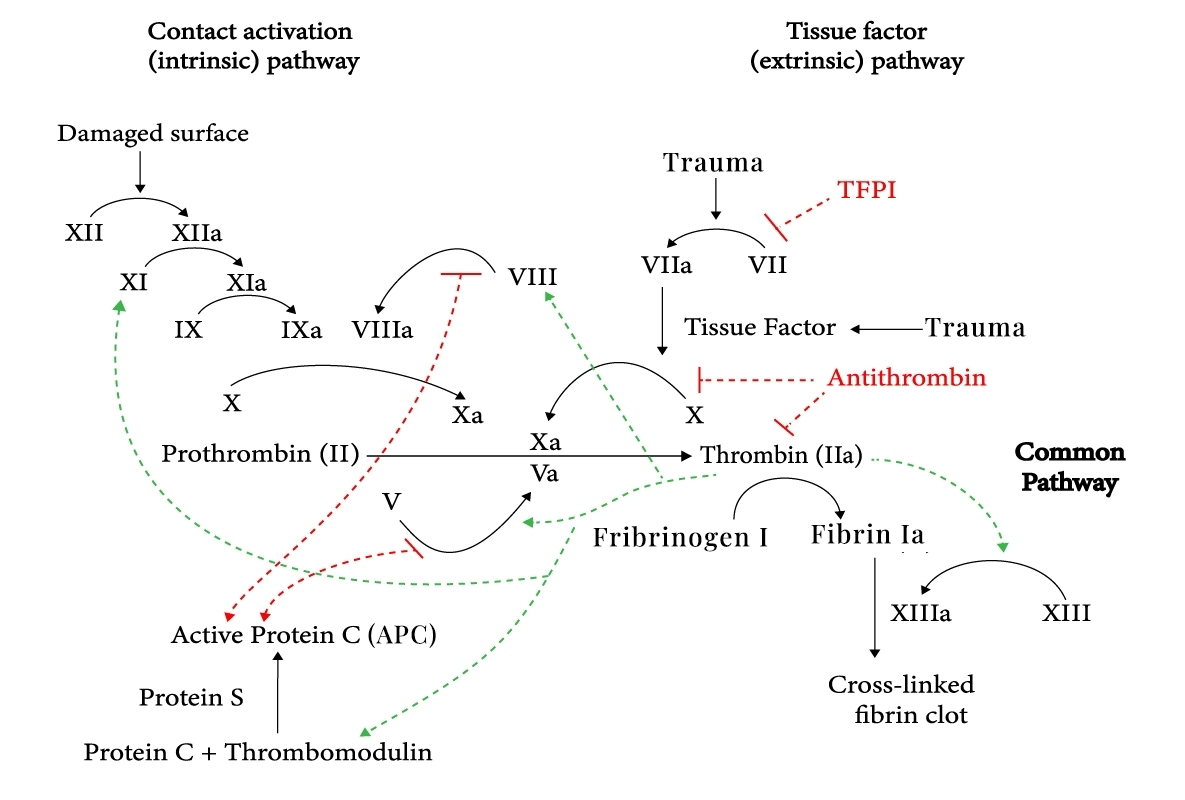
SR604 is an affinity matured and humanized anti-activated protein C (APC) monoclonal antibody, selectively blocks FVa/FVIIIa degradation by inhibition of APC anticoagulant function, promote hemostatic balance toward clots formation in the surface of vasculature. While SR604 and its parent antibody HAPC1573 preserve APC normal cytoprotective function including endothelial cell barrier protection, and anti-inflammatory effects.
SR604 is being developed for routine prophylaxis via subcutaneous (SC) injections to prevent or reduce the frequency of bleeding episodes in adult and pediatric patients with hemophilia A and B with or without inhibitors and other coagulant factor deficiencies.
In May 2023, Equilibra Bioscience received FDA Orphan Drug Designation (ODD) for recombinant anti-human activated protein C humanized monoclonal antibody (SR604) for the treatment of Hemophilia A and Hemophilia B.
EXPANDED ACCESS POLICY
Equilibra Bioscience is a clinical stage biopharmaceutical company committed to the development of safe and effective novel therapies for severe and life-threatening orphan bleeding disorders and providing a better life for patients with bleeding disorders. In February 2024, The U.S. Food and Drug Administration (FDA) has cleared the company’s Investigational New Drug (IND) Application for SR604. In May 2024, FDA granted Fast Track Designation (FTD) to SR604 for the routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adults with severe hemophilia A and severe hemophilia B with or without inhibitors. Phase 1 study of SR604 is being conducted following approvals of the IND application, started with enrollment of healthy participants and will expand to participants with Hemophilia A or Hemophilia B, with or without inhibitors. You can find additional information by accessing https://clinicaltrials.gov (NCT06349473).
Expanded Access is a potential pathway for a patient with an immediately life-threatening condition or serious disease or condition to gain access to an investigational drug for treatment outside of a clinical trial when no comparable or satisfactory alternative therapy exists.
As a general policy, Equilibra Bioscience will not provide SR604 on Expanded Access at this time anywhere in the world. Equilibra Bioscience believes that participation in our clinical trial program is the most appropriate way to access our investigational therapy. Equilibra will continue to assess the eligibility requirements and criteria for Early Access and will re-evaluate this policy from time to time.
Information Links:
- Equilibra Bioscience received FDA Orphan Drug Designation (ODD) for recombinant anti-human activated protein C humanized monoclonal antibody (SR604) for the treatment of Hemophilia A and Hemophilia B. NEWS
- Safety and efficacy of an anti–human APC antibody for prophylaxis of congenital factor deficiencies in preclinical models. Blood, 2023;142(12):1071-1081.
- Blocking human protein C anticoagulant activity improves clotting defects of hemophilia mice expressing human protein C. Blood Advances, 2022;6(11):3304-3314.

- Targeted inhibition of activated protein C by a non-active-site inhibitory antibody to treat hemophilia. Nature Communications,
2020;11(1):2992.
RESOURCES
the rare bleeding disorders

NORD

NBDF